When Testing Fails: The Hidden Risks of Healthcare Surfaces
Every five minutes, a patient dies from a healthcare-associated infection (HAI). According to the U.S. Centers for Disease Control and Prevention, one in 31 patients will acquire an HAI during their hospital stay.
For the experts who participated in ISSA Healthcare’s recent webinar, The Truth About Testing: Addressing the Inconsistencies of Healthcare Surface Materials, these numbers are more than statistics—they are a call to action.
Hosted by the Healthcare Surfaces Institute (HSI), the panel brought together scientists, engineers, and infection prevention professionals to confront a complex reality: The data we rely on to evaluate medical devices and surface materials is often inconsistent, incomplete, or unreliable.
The unseen risks of healthcare surfaces
Barbara Strain, principal of Barbara Strain Consulting and a microbiologist, opened the discussion by reminding the audience of the importance of the issue. “Every five minutes, a patient dies from a healthcare-associated infection. One in 31 patients will acquire a healthcare-associated infection. Let that set in … because that’s the bell weather of why we’re here and why we’re passionate about going the nth degree of how surfaces play a part in this phenomenon.”
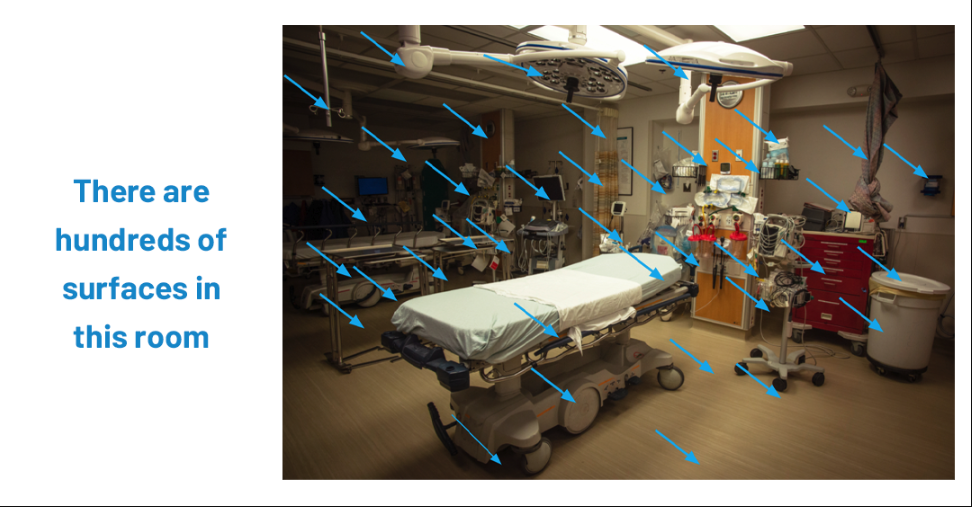
Strain emphasized that while hand hygiene and cleaning protocols receive attention, the actual surfaces of medical devices—such as monitors, infusion pumps, glucose meters, beds, and more—remain a hidden threat. When cleaning chemicals cause “micro damage” to plastics and coatings, bacteria and fungi can take root, forming biofilms that resist disinfectants and continue spreading to patients.
Recalls and damage: A frontline view
For Ray Laxton, executive director of biomedical engineering at Piedmont Healthcare, the impact of surface damage is painfully familiar. “In 2024 alone, there were 13 related recalls… In our system, we recently had a recall on our infusion devices. Greater than 15,000 devices were impacted. Every hospital in the system and almost every clinical unit because infusion pumps are everywhere.”
That recall triggered an eight-month process of pulling pumps out of service, cleaning, swapping, shipping, and documenting—a process that disrupted patient care and increased risks. Beyond recalls, Laxton described how cleaning agents cause physical damage: brittle covers, cracked entry pads, and broken doors that trap microbes.
The material science challenge
Jim Hicks, a customer technical development leader at a global materials company, explained why the interaction of disinfectants with polymers is so unpredictable. “It’s fairly easy to address very well-known engineering attributes like strength and stiffness … Chemical resistance has always been a more nebulous, maybe a little bit less known factor that the device makers do not quite understand how to compare and test with.”
Unlike sterilization methods, which follow well-established protocols, chemical disinfectants vary widely in their formulations. “Although it’s a similar method, we don’t always test the same way. And so it’s very difficult for a device maker to understand how one material compares to another.”
Ellen Turner, director of marketing and business development at Andronico, added that too many manufacturers default to familiar polymers instead of exploring better alternatives. “Scientists at the beginning of the value chain that develops polymers… need to be talking to the scientists who are touching the patient. If that connection isn’t made, somebody loses—and what we want is for everybody to win even all the way to the patient.”
From inconsistency to innovation: A patented solution
A decade ago, HSI gathered a team of competitors—polymer suppliers, disinfectant manufacturers, and device engineers—to confront the testing inconsistency problem. That collaboration resulted in a patented test method designed to bring transparency and reliability to the evaluation of materials.
Mark Lamont, director of mechanical engineering at Zebra Technologies, recalled the process: “One of the first things that we all spent a lot of time discussing was understanding the different types of uses within the healthcare industry… and what is the best way that we can come up with for a common test methodology that would be used throughout the industry.”
Hicks described the unusual spirit of cooperation. “There were multiple polymer suppliers who are normally competing against each other and… it was no competition, we saw the need, we actually took a great deal of pleasure in talking together about the common issues we see.”
Turner called it “extremely exciting,” noting that the collaboration showed how science can transcend competition when patient safety is at stake.
Designing with the use case in mind
Panelist Jada Lindo, a mechanical engineer at Zebra Technologies, emphasized that testing must reflect real-world conditions. “When designing our devices, it’s really important that we do a deep dive in understanding our customer’s use case… not only focusing on the environmental conditions but also understanding how the customer is really interacting with that device.”
Lamont added that without consistent standards, manufacturers are left developing their own tests, which leads to varying instructions for use (IFUs) and inconsistent cleaning guidance. Currently, no regulatory body enforces a uniform reporting method.
What a standard could change
The panel agreed that a universally accepted test method would transform the industry within five years. Lamont explained that such a standard would “guide and direct the research and development that’s done in investigating new materials… to something that’s more aligned with what the health care industry is looking for.”
Laxton noted the practical benefit: “Right now when we’re looking to purchase new equipment, we don’t focus a lot on this particular issue. Having a standard out there… would make it much easier for us to include that in our requirements.”
Barriers, blind spots, and parallels
What stands in the way of adoption? Competing opinions, limited awareness, and resistance to change. But as Hicks pointed out, “It just needs to be shared to the industry, to the device makers and have them start asking for it.”
Audience questions raised useful comparisons. Lamont pointed to cell phone manufacturing, where ingress protection (IP) standards now define dust and water resistance. Turner drew from heavy industry, where collaborative testing ensures equipment can withstand corrosive chemicals. Both examples show how healthcare could benefit from the same kind of cross-sector discipline.
When asked about blind spots, Strain stressed the need for education. Too often, healthcare staff think only of obvious items like beds and tables, overlooking the multiple surface materials in a single medical device. Without proper validation, “we’re in a cycle of never-ending change.”
The call to action
As the webinar drew to a close, panelists returned to a common theme: Collaboration, education, and accountability across the value chain. Strain urged attendees to “develop your whole culture of safety. Don’t overlook surfaces and how they are designed for durability, reliability, and compatibility … Thoroughly vet the cleaning and disinfecting section of the instructions for use well before purchasing.”
Turner was even more direct: “This is a cost issue, it’s a safety issue and everyone, regardless of the severity of the pain point for you, should act.”
The Healthcare Surfaces Institute, now part of ISSA Healthcare, continues to build task forces, push for regulatory recognition, and expand its patented methods into assembled medical devices. But as the panelists made clear, progress depends on whether manufacturers, healthcare providers, and infection prevention specialists demand consistent, reliable testing.
Because behind every test method, every polymer, and every device is a patient whose safety depends on getting it right.
Learn more about ISSA Healthcare and its contributions to the cleaning industry and the organizations it serves at issa.com/healthcare.